Cancer
A phase 1 safety and feasibility trial of a ketogenic diet plus standard of care for patients with recently diagnosed glioblastoma
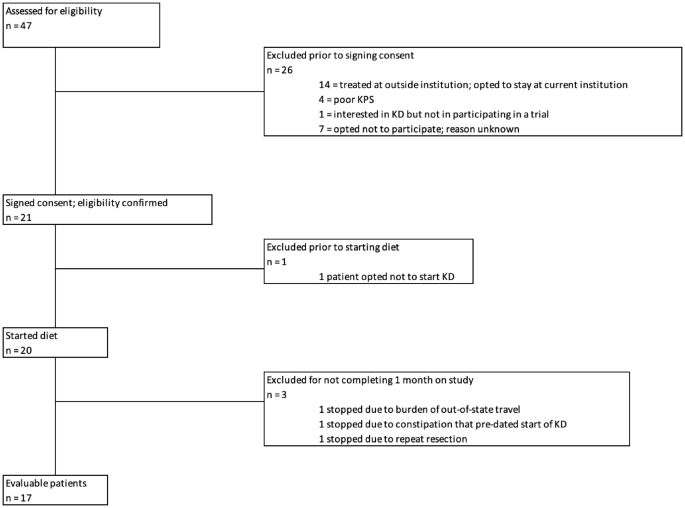
A phase 1, single-arm clinical trial investigated the safety and feasibility of implementing a ketogenic diet (KD) alongside standard-of-care chemoradiation in patients with newly diagnosed glioblastoma (GBM), a highly aggressive brain cancer with poor prognosis. In addition to safety endpoints, the study also explored progression-free survival (PFS) and overall survival (OS) as secondary outcomes.
17 patients were placed on a medically supervised KD during concurrent radiation and temozolomide chemotherapy. They began a dietitian-supervised ketogenic diet with a 3:1 ratio of fat to protein-and-carbohydrate. Each patient received an individualized nutrition plan and ongoing support from a clinical dietitian, with the goal of inducing and maintaining nutritional ketosis (β-hydroxybutyrate ≥0.3 mmol/L). Patients measured blood glucose and ketone levels twice daily, with results monitored remotely by the clinical team.
Key Findings
- Feasibility and adherence:
- All 17 participants completed the full 6-month dietary intervention
- The majority maintained nutritional ketosis throughout treatment
- Safety:
- No serious adverse events were attributed to the ketogenic diet
- Reported side effects included mild gastrointestinal symptoms, manageable with supportive care
- Survival outcomes:
- Median progression-free survival (PFS): 12.9 months
- Median overall survival (OS): 29.4 months
- Both were measured from diet initiation, which began at a median of 56 days after diagnosis. For context, the landmark EORTC/NCIC Phase 3 trial that established the existing standard of care reported PFS of 6.9 months and OS of 14.6 months from time of diagnosis.
- Metabolic effects:
- Most participants maintained stable body weight
- Blood glucose levels remained well controlled, with β-hydroxybutyrate levels typically in the target range (≥0.3 mmol/L on >50% of study days)
- Quality of life:
- No evidence of deterioration in quality of life or functional status due to the diet
This Phase 1 trial demonstrates that a ketogenic diet can be safely integrated with standard chemoradiation in patients with newly diagnosed glioblastoma. The intervention was feasible and well tolerated, with no diet-related serious adverse events. It also highlights the potential of combining dietary interventions with standard cancer therapy, using remote monitoring to support adherence and safety. Finally, it lays the foundation for an ongoing multicenter randomized trial to evaluate its efficacy.