Cancer
Targeting fatty acid oxidation enhances response to HER2-targeted therapy

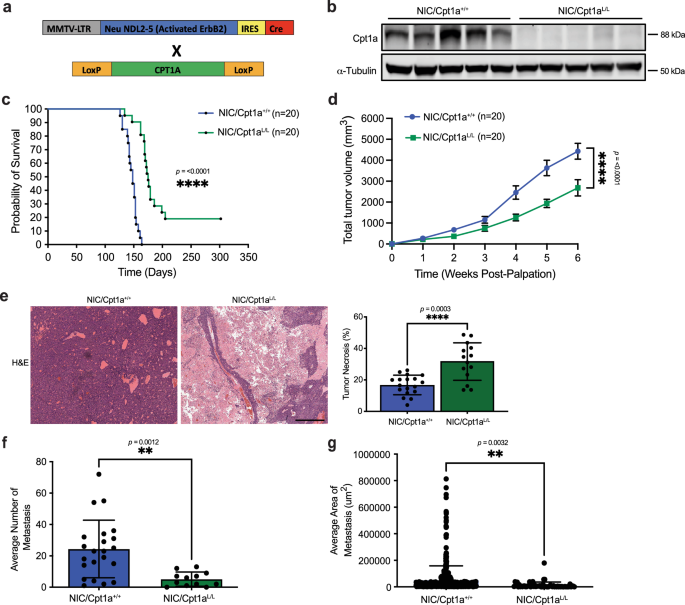
In a recent article in Nature Communications, the authors explore the complexity of metabolic reprogramming in ErbB2+ breast cancer models, in vitro and in vivo. While the Warburg effect shows increased glucose consumption in tumors, recent research shows that HER2+ breast cancer cells can rewire lipid metabolism to fuel their growth and resist treatments.
Carnitine palmitoyl transferase 1a (Cpt1a) is a key enzyme involved in the mitochondrial uptake and oxidation of long-chain fatty acids, with a crucial role in fatty acid metabolism. In ErbB2+ breast cancer models, removing Cpt1a reduced tumor growth and metastasis. However, these cells also became more dependent on glucose, which helped them survive and grow.
In this study the combination of Cpt1a inhibition with either a ketogenic diet (high in long-chain fatty acids) or treatment with anti-HER2 monoclonal antibodies significantly affected tumor growth, enhanced apoptosis, and reduced lung metastasis. These findings offer a new potential therapeutic approach to combat resistance in HER2+ breast cancer.