Musculoskeletal
β-hydroxybutyrate suppresses pathological changes of blood-induced arthropathy in rats
Blood-induced arthropathy is a debilitating complication in haemophilia, leading to joint inflammation, fibrosis, and long-term damage. The ketone body β-hydroxybutyrate (BHB) has been hypothesized to protect joints from progressive damage.
Study Design:
- Aim: To investigate the relationship between circulating blood BHB levels and the extent of inflammation and subsequent damage in joints
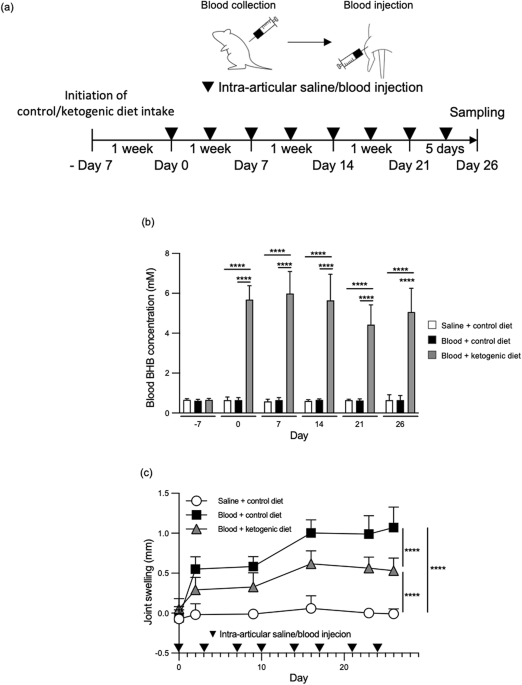
- Animal Model: Rats received repeated intra-articular blood injections to simulate bleeding-induced joint damage. A ketogenic diet was used to elevate BHB levels, and its effects on joint inflammation and tissue fibrosis were assessed.
- Cell Study: Blood-exposed macrophages (THP-1 cell line) were treated with BHB to evaluate its impact on inflammatory markers.
Key Findings:
- Reduced joint swelling: Rats fed a ketogenic diet had significantly lower joint swelling compared to controls.
- Less tissue fibrosis: Periarticular fibrosis was reduced in BHB-supplemented rats, suggesting protection against joint damage.
- Suppressed inflammation: BHB lowered IL-1β levels, a key inflammatory cytokine driving arthropathy progression.
- Macrophage modulation: BHB-treated macrophages showed reduced inflammatory response, further supporting its protective role.
Conclusion:
These findings suggest that BHB may protect joints from blood-induced damage by reducing inflammation and fibrosis, offering a potential therapeutic strategy for haemophilia-related arthropathy. Further clinical studies are needed to explore ketogenic diets or oral BHB supplementation as non-invasive interventions to improve joint health and quality of life in people with haemophilia.